
We have had the pleasure of speaking to customers about a whole manner of interesting topics this week. So, this time, I would like to answer a few questions that have been raised during these conversations. Perhaps they will help you too. Let’s get started…
1. Plating over zinc - why is it so difficult?

Many people have had issues plating over zinc before and there is a good reason for this. Older zinc was quite often poorly cast, resulting in gas bubbles and despite the metal being a solid, these gas bubbles still travel very slowly within the metal over the course of decades. This results in a metal that can emit gas when plated with certain other metals such as nickel, causing blistering.
The way to get around this, is to give the zinc a good ten-minute plate in copper strike which seals off the zinc. The copper strike will have a muddy appearance, but do not worry – half an hour in the electroforming tank and it will be super shiny, ready to be nickel plated, and then gold plated.
Pitted zinc can be a problem because it is tempting to grind it back to remove the pits. The trouble is, due to the honeycomb nature of zinc (and its gas bubbles), you may be revealing larger pits by grinding it back. So, on particularly old zinc, you may need to fill the holes rather than trying to grind them away, solder is often used for this.
2. Brown powder is present on my gold plated surface - what does this mean?

If you have gold plated an item, either by brush or tank, and you have used a lot of gold, you may occasionally find what looks like a dark brown powder on the surface instead of shiny gold. This wipes off very easily with some tissue paper and G.S.P Gold Polish and it is nothing more than a slight carbon build-up from your probe or anode.
If you are tank plating, the use of air agitation should prevent this (air agitation is always recommended for gold tank plating anyway). If you are brush plating, then it is less avoidable but nothing to worry about, there is still shiny gold underneath the powder.
3. Nickel tank plating and magnets
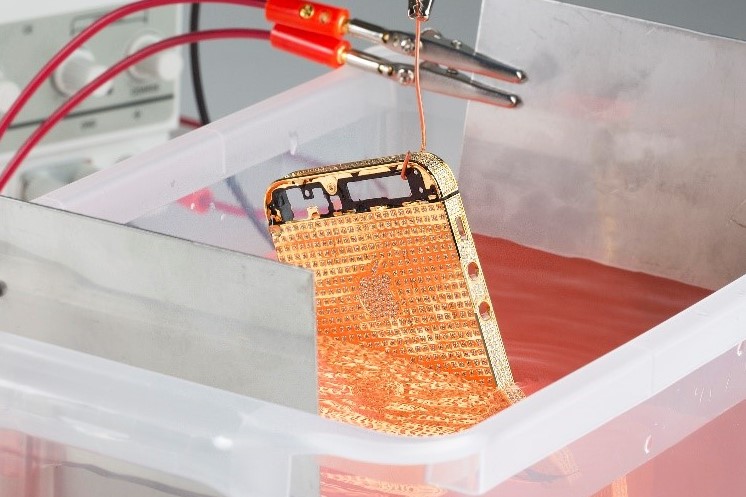
I have been asked on occasion to plate some aluminium phone cases which are held together by magnets. After polishing and treating with G.S.P AllyCu, I then plated them in nickel for 40 minutes, as is the usual process. What I didn’t factor in, was the fact that nickel is ferro-magnetic, and this resulted in the nickel plate around the magnets seeming to take on a wrinkled appearance in the direction of the magnetic field. This gave me the revelation that if you plate items with magnets, it is best to remove the magnets where possible before plating.
Please note: our G.S.P 24K Gold Plating Solution and our G.S.P 24K Tank Gold Plating Solution are affected by magnets even though gold itself is not magnetic. The hardeners in these solutions are cobalt-based and so magnets can still influence the plating.
To wrap things up, be sure to treat your zinc to a copper strike bath for blister-free plating, remove magnets where possible when plating with nickel and wipe away carbon build-up on gold plate by using our G.S.P Gold Polish.
As always, I hope that these tips are useful, please get in touch with questions and your topic suggestions for future blog posts. Until next time!

